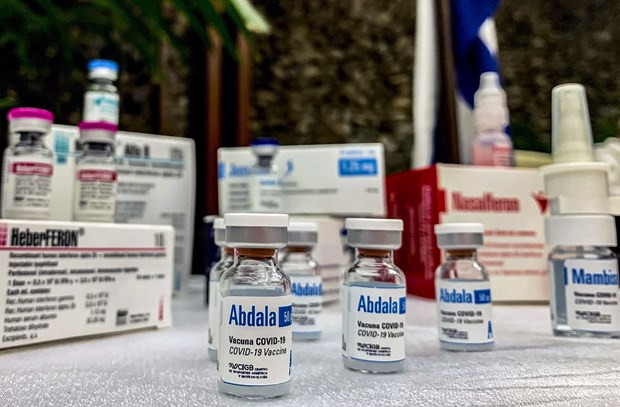
 The Ministry of Health approves with conditions for the emergency use of Cuba's Abdala. (Photo: AFP/VNA)
The Ministry of Health approves with conditions for the emergency use of Cuba's Abdala. (Photo: AFP/VNA)The vaccine by manufactured at the AICA Laboratories Company and Base Business Unit (BBU) AICA in Cuba.
The Centre for Immunisation Vaccines Polyvac in Vietnamasked for permission for the vaccine to be used.
The MoH insisted on a number of conditions beforegranting approval.
It has stated that the Drug Administration of Vietnamis responsible for licensing Abdala vaccine based on regulations forimportation and quality management.
The Department of Science, Technology and Training hasbeen given responsibility for selecting a unit that is qualified to evaluatethe vaccine’s safety and effectiveness.
The General Department of Preventive Medicine is incharge of injecting the vaccine.
And finally, the National Institute for Control ofVaccine and Biologicals is responsible for verifying and issuing certificatesfor the vaccine before it can be used./.