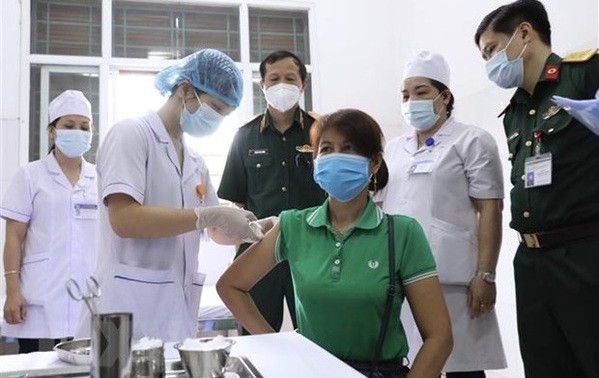
 Injecting the second shot of Nano Covax vaccine to a volunteer in Van Lam district, Hung Yen province (Photo: VNA)
Injecting the second shot of Nano Covax vaccine to a volunteer in Van Lam district, Hung Yen province (Photo: VNA)They are among 13,000 volunteers involving in the third trial phase, 1,000 of whom already received the second doses on July 22.
Lieutenant Colonel Associate Prof. Dr. Chu Van Men, Director of the Center forClinical Trials and Bioequivalence under the Military Medical University, said that all the volunteers, receiving onlythe 25mcg doses, are in stable health conditions.
Nano Covax has been developed by the Nanogen PharmaceuticalBiotechnology JSC based on recombinant DNA/protein technology from May 2020.
It went through the first phase trial from December 18,2020, and the second phase from February 26, 2021. The third phase started onJune 11, 2021.
Results from the first two trial phases showed that all volunteers developedantibodies against SARS-CoV-2.
The Ministry of Health and the NationalCouncil of Ethics in Biomedical Research on June 25 agreedwith the Pasteur Institute in Ho Chi Minh City and the Military MedicalUniversity to speed up the third-phase clinical trial of thevaccine in which volunteers were expected to have received the first jabs byJuly 15 and the second shots by August 15.
Vietnam has so far approved six vaccines for emergency use,namely Janssen, Moderna, Sputnik V, Pfizer, Sinopharm and AstraZeneca.
The Government is making efforts to secure at least 150 million vaccine dosesto inoculate 70 percent of the population.
On July 27 morning, Vietnam confirmed an additional 2,764COVID-19 cases, raising the national total to 109,111. The number of casesreported since the fourth coronavirus wave hit the country in late Aprilreached 105,338./.